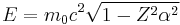Extended periodic table
There are currently seven periods in the periodic table of chemical elements, culminating with atomic number 118. If further elements with higher atomic numbers than this are discovered, they will be placed in additional periods, laid out (as with the existing periods) to illustrate periodically recurring trends in the properties of the elements concerned. Any additional periods are expected to contain a larger number of elements than the seventh period, as they are calculated to have an additional so-called g-block, containing 18 elements with partially filled g-orbitals in each period. An eight-period table containing this block was suggested by Glenn T. Seaborg in 1969.[1]
No elements in this region have been synthesized or discovered in nature. (Element 122 was claimed to exist naturally in April 2008, but this claim was widely believed to be erroneous.)[2] The first element of the g-block may have atomic number 121, and thus would have the systematic name unbiunium. Elements in this region are likely to be highly unstable with respect to radioactive decay, and have extremely short half lives, although element 126 is hypothesized to be within an island of stability that is resistant to fission but not to alpha decay. It is not clear how many elements beyond the expected island of stability are physically possible, if period 8 is complete, or if there is a period 9. If period 9 does exist, it is likely to be the last.
According to the orbital approximation in quantum mechanical descriptions of atomic structure, the g-block would correspond to elements with partially-filled g-orbitals. However, spin-orbit coupling effects reduce the validity of the orbital approximation substantially for elements of high atomic number.[3]
Contents |
Extended periodic table, including the g-block
| 1 | 1 H |
2 He |
||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne |
||||||||||||||||||||||||||||||||||||||||||
| 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar |
||||||||||||||||||||||||||||||||||||||||||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr |
||||||||||||||||||||||||||||||||
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
||||||||||||||||||||||||||||||||
| 6 | 55 Cs |
56 Ba |
57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
||||||||||||||||||
| 7 | 87 Fr |
88 Ra |
89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Uut |
114 Uuq |
115 Uup |
116 Uuh |
117 Uus |
118 Uuo |
||||||||||||||||||
| 8 | 119 Uue |
120 Ubn |
121 Ubu |
122 Ubb |
123 Ubt |
124 Ubq |
125 Ubp |
126 Ubh |
127 Ubs |
128 Ubo |
129 Ube |
130 Utn |
131 Utu |
132 Utb |
133 Utt |
134 Utq |
135 Utp |
136 Uth |
137 Uts |
138 Uto |
139 Ute |
140 Uqn |
141 Uqu |
142 Uqb |
143 Uqt |
144 Uqq |
145 Uqp |
146 Uqh |
147 Uqs |
148 Uqo |
149 Uqe |
150 Upn |
151 Upu |
152 Upb |
153 Upt |
154 Upq |
155 Upp |
156 Uph |
157 Ups |
158 Upo |
159 Upe |
160 Uhn |
161 Uhu |
162 Uhb |
163 Uht |
164 Uhq |
165 Uhp |
166 Uhh |
167 Uhs |
168 Uho |
| 9 | 169 Uhe |
170 Usn |
171 Usu |
172 Usb |
173 Ust |
|||||||||||||||||||||||||||||||||||||||||||||
Blocks of the periodic table
| s-block | p-block | d-block | f-block | g-block |
(Undiscovered (theorized) elements are coloured in a lighter shade)
All of these hypothetical undiscovered elements are named by the International Union of Pure and Applied Chemistry (IUPAC) systematic element name standard which creates a generic name for use until the element has been discovered, confirmed, and an official name approved.
The positioning of the g-block in the table (to the left of the f-block, to the right, or in between) is speculative. The positions shown in the table above corresponds to the assumption that the Madelung rule will continue to hold at higher atomic number; this assumption may or may not be true. At element 118, the orbitals 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, 5p, 5d, 5f, 6s, 6p, 6d, 7s and 7p are assumed to be filled, with the remaining orbitals unfilled. The orbitals of the eighth period are predicted to be filled in the order 8s, 5g, 6f, 7d, 8p. However, after approximately element 120, the proximity of the electron shells makes placement in a simple table problematic; for example, calculations suggest that it may be elements 165 and 166 which occupy the 9s block (leaving the 8p orbital incomplete) assuming they are physically possible.[5]
End of the periodic table
The number of physically possible elements is unknown. The light-speed limit on electrons orbiting in ever-bigger electron shells theoretically limits neutral atoms to a Z of approximately 173,[6] after which it would be nonsensical to assign the elements to blocks on the basis of electron configuration. However, it is likely that the periodic table actually ends much earlier, possibly soon after the island of stability,[7] which is expected to center around Z = 126.[8]
Additionally the extension of the periodic and nuclides tables is restricted by the proton drip line and the neutron drip line.
Bohr model breakdown
The Bohr model exhibits difficulty for atoms with atomic number greater than 137, for the speed of an electron in a 1s electron orbital, v, is given by
where Z is the atomic number, and α is the fine structure constant, a measure of the strength of electromagnetic interactions.[9] Under this approximation, any element with an atomic number of greater than 137 would require 1s electrons to be traveling swifter than c, the speed of light. Hence a non-relativistic model such as the Bohr model is inadequate for such calculations.
The Dirac equation
The semi-relativistic Dirac equation also has problems for Z > 137, for the ground state energy is
where m0 is the rest mass of the electron. For Z > 137, the wave function of the Dirac ground state is oscillatory, rather than bound, and there is no gap between the positive and negative energy spectra, as in the Klein paradox.[10] Richard Feynman pointed out this effect, so the last element expected under this model, 137 (untriseptium), is sometimes called feynmanium.
However, a realistic calculation has to take into account the finite extension of the nuclear-charge distribution. This results in a critical Z of ≈ 173 (unseptrium), such that non-ionized atoms may be limited to elements equal to or lower than this.[6]
See also
- Electron configuration
- Nuclear shell model
- Table of nuclides (combined)
References
- ↑ [1]
- ↑ "Heaviest element claim criticised". Rsc.org. 2008-05-02. http://www.rsc.org/chemistryworld/News/2008/May/02050802.asp. Retrieved 2010-03-16.
- ↑ For example, an element in the column labeled g1 may indeed have exactly one valence-shell g-electron (as the name suggests), but it is also possible that it would have more, or none at all.
- ↑ The labels "g1", etc. are inspired by the Madelung rule, but this is merely an empirical rule, with well-known exceptions such as copper.
- ↑ Pekka Pyykkö, Peter Schwerdtfeger (2004), Relativistic electronic structure theory, p 23.
- ↑ 6.0 6.1 Walter Greiner and Stefan Schramm (2008). American Journal of Physics 76: 509. doi:10.1119/1.2820395., and references therein.
- ↑ Encyclopædia Britannica. "transuranium element (chemical element) - Britannica Online Encyclopedia". Britannica.com. http://www.britannica.com/EBchecked/topic/603220/transuranium-element. Retrieved 2010-03-16.
- ↑ S. Cwiok, P.-H. Heenen and W. Nazarewicz (2005). "Shape coexistence and triaxiality in the superheavy nuclei". Nature 433: 705.
- ↑ See for example R. Eisberg and R. Resnick, Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles, Wiley (New York: 1985).
- ↑ James D. Bjorken and Sidney D. Drell, Relativistic Quantum Mechanics, McGraw-Hill (New York:1964).
External links
- Images of g-orbitals from the University of Kentucky
- jeries.rihani.com - The extended periodic table of the elements.
- Eric Scerri, The Periodic Table, Its Story and Its Significance, Oxford University Press, 2007.
|
||||||||||||||||||||